Sinopharm Vaccine Registration
Registration for the first dose of Sinopharm vaccine starts 12 pm. The Sinovac vaccine in particular is extremely popular with nearly 100000 doses administered to date.

Vietnam Licenses Firm To Import 5 Mln Doses Of Sinopharm Vaccine Reuters
Rationale COVID-19 is worldwide pandemic devastated various countries.

Sinopharm vaccine registration
. Malaysias health ministry said on Friday Jul 16 it has granted conditional approval for emergency use to the COVID-19 vaccine manufactured by Chinas Sinopharm. The registration period for the Sinopharm vaccine went from June 14 to June 18. What you need to know. Meanwhile Nithi Mahanonda CRA secretary-general posted on his Facebook page that another 1 million vaccine doses had been delivered to Thailand.The WHO issued its emergency use listing for the Sinopharm vaccine on May 7 2021 some 4 months after Chinas National Medical Products Administration authorized it on December 31 2020. The HSA has approved 11 applications for private hospitals and clinics to import Chinas Sinopharm vaccine under the special access route. Covid-19 vaccine is a mean to curtail. Raffles Medical Group asked people to register their interest in the Sinopharm vaccine in a Facebook post on Monday.
The registration opened on Sunday at 8am through the website and CRA SINOP. According to the WHO a large multi-country phase three. CRAs Sinopharm jabs registration is aimed at 40000 citizens in 10 red zone provinces. The following groups can get the vaccine by visiting one of SEHAs vaccination centres without prior appointment.
Here is what you need to know. It is approved for use in those aged 18 and above. DUBAI Reuters - Bahrain said on Sunday it had approved a COVID-19 vaccine developed by China National Pharmaceutical Group Sinopharm and launched online registration for the vaccine for. Of the 100 million Chinese vaccines half will be provided by Sinopharm and half by Sinovac with deliveries planned for July to September 2021 a WHO document dated July 29 says.
The Sinopharm COVID-19 vaccine. Those registered can already receive their first doses of the vaccine on Monday July 5. Sinopharm Vaccine Approved By HSA Under Special Access Route. Sinopharms COVID-19 vaccine.
Under the special access route SAR Raffles Medical Group has received approval to import the Sinopharm vaccines. On Johnson Johnsons Janssen Covid-19 vaccine Dr Noor Hisham said the vaccine which is also known as Janssen Covid-19 Vaccine Suspension for Injection is manufactured by Belgium-based Janssen Pharmaceutica NV. The Sinopharm vaccine is administered in two doses given three to four weeks apart. The Straits Times.
On 9 December 2020 Ministry of Health and Prevention MOHAP announced that it had officially registered the Beijing Institute of Biological Products inactivated vaccine known as Sinopharm vaccine which was developed jointly with Sinopharm -. The CRA announced on Saturday June 19 that 17 070 organizations and companies nationwide have registered to obtain the Sinopharm vaccine to inoculate more than 487 million employees. Al Owais noted that the approved Sinopharm vaccine is the same vaccine that was used in the groundbreaking 4Humanity trials the first global clinical Phase III trial of an inactivated vaccine to combat COVID-19 which was launched in UAE in July this year and later was officially registered by the UAE Ministry of Health and Prevention MOHAP on 9th December. COVID-19 Vaccination Updated Sinopharm Vaccine Guidelines Objective To provide guidelines for the Sinopharm vaccine storage handling administration and safe disposal along with recommendations for vaccine recipients.
Even though the Pfizer-BioNTech and Moderna vaccines are available for free under our national vaccination programme some may look for alternative options. You may register your interest and you will be notified on next steps once the vaccine is available. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group. Sinopharm and Pfizer- BioNTech vaccines are available for citizens and residents for free Those who wish to get the should book prior appointment in one of the vaccination centers below.
He said Malaysias conditional registration approval for the use of the Sinopharm vaccine has been given to Duopharma M Sdn Bhd. Over 14000 organisations register for Sinopharms vaccine The Chulabhorn Royal Academy CRA announced on Friday that 14634 organisations had applied to get Sinopharms Covilo vaccine to inoculate 4545272 staff as an alternative to the governments Sinovac and AstraZeneca vaccines. Download the COVID-19 Vaccine Pre-Registration Forms. The registration is available via bookingmohgovge as well as the health ministry hotline 1522.
WHO approves Sinopharm vaccine for emergency use in all countries.
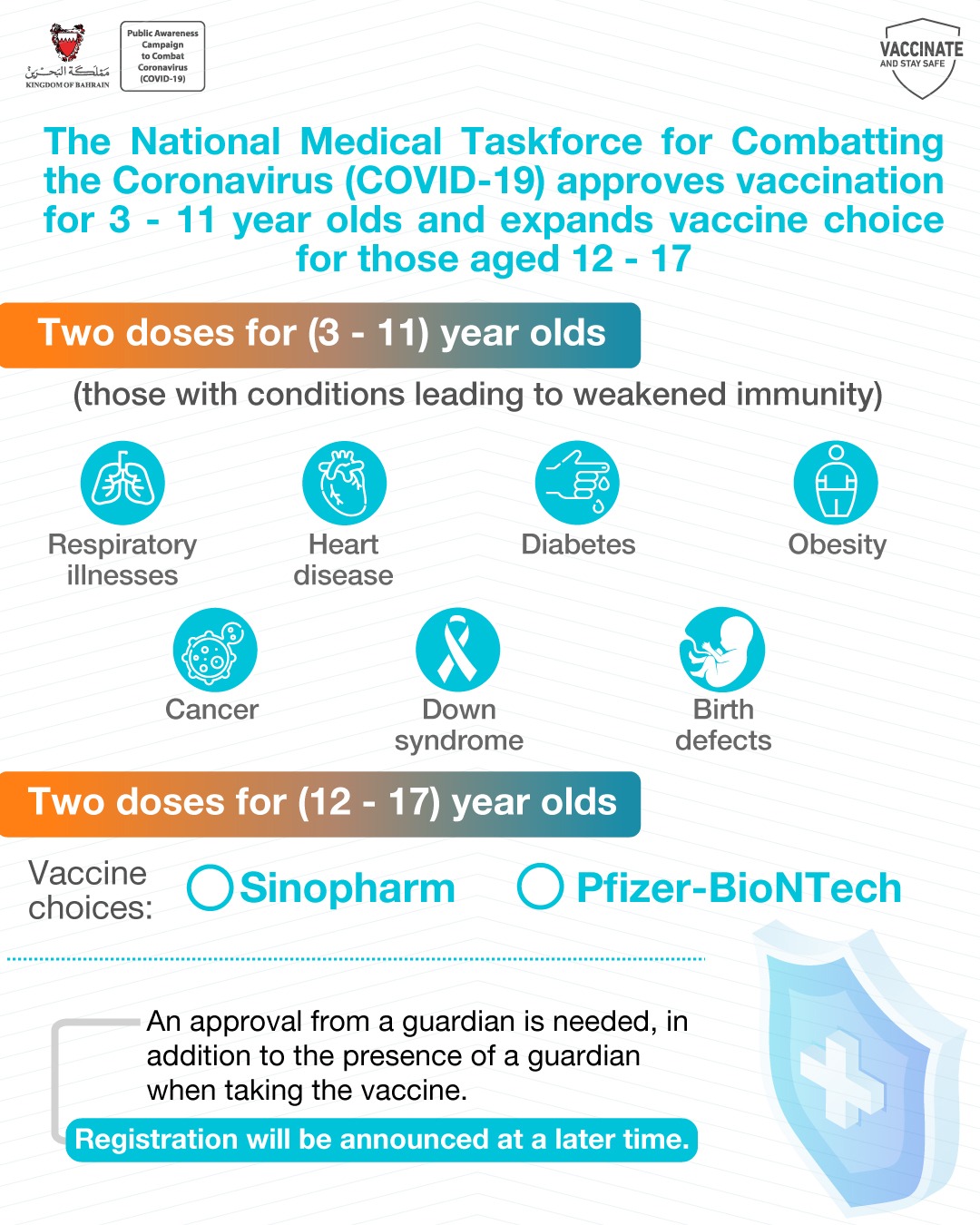
Covid 19 Taskforce Approves Vaccination For 3 11 Year Olds And Expands Vaccine Choice For Those Aged 12 17 The Official Website For The Latest Health Developments Kingdom Of Bahrain
/cloudfront-us-east-2.images.arcpublishing.com/reuters/K7IMX7V5NZOOVGSOPKVPNUA6MY.jpg)
Uae Bahrain To Offer Sinopharm Covid 19 Booster Shots Reuters
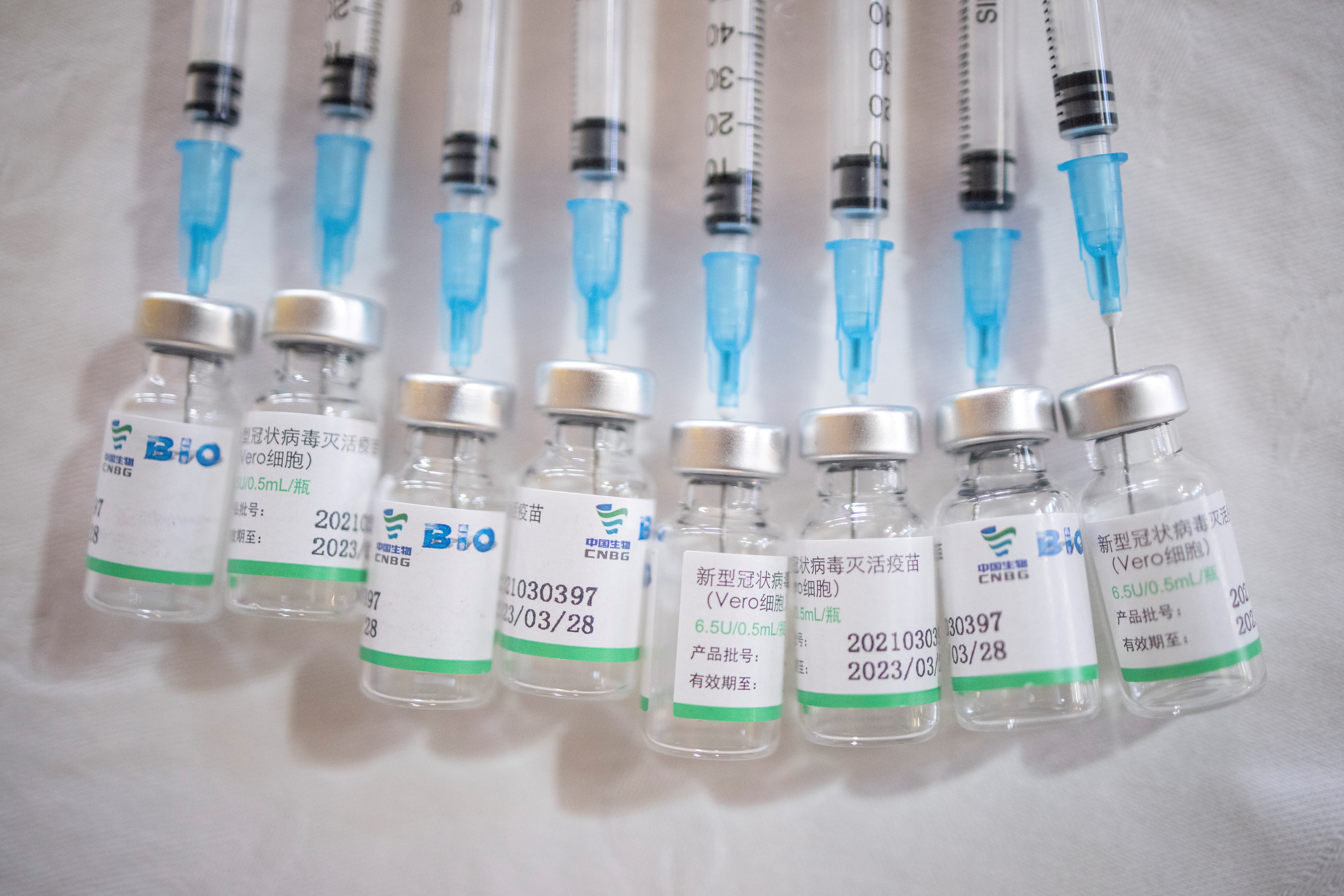
Uae Bahrain To Offer Sinopharm Covid 19 Booster Shots Reuters
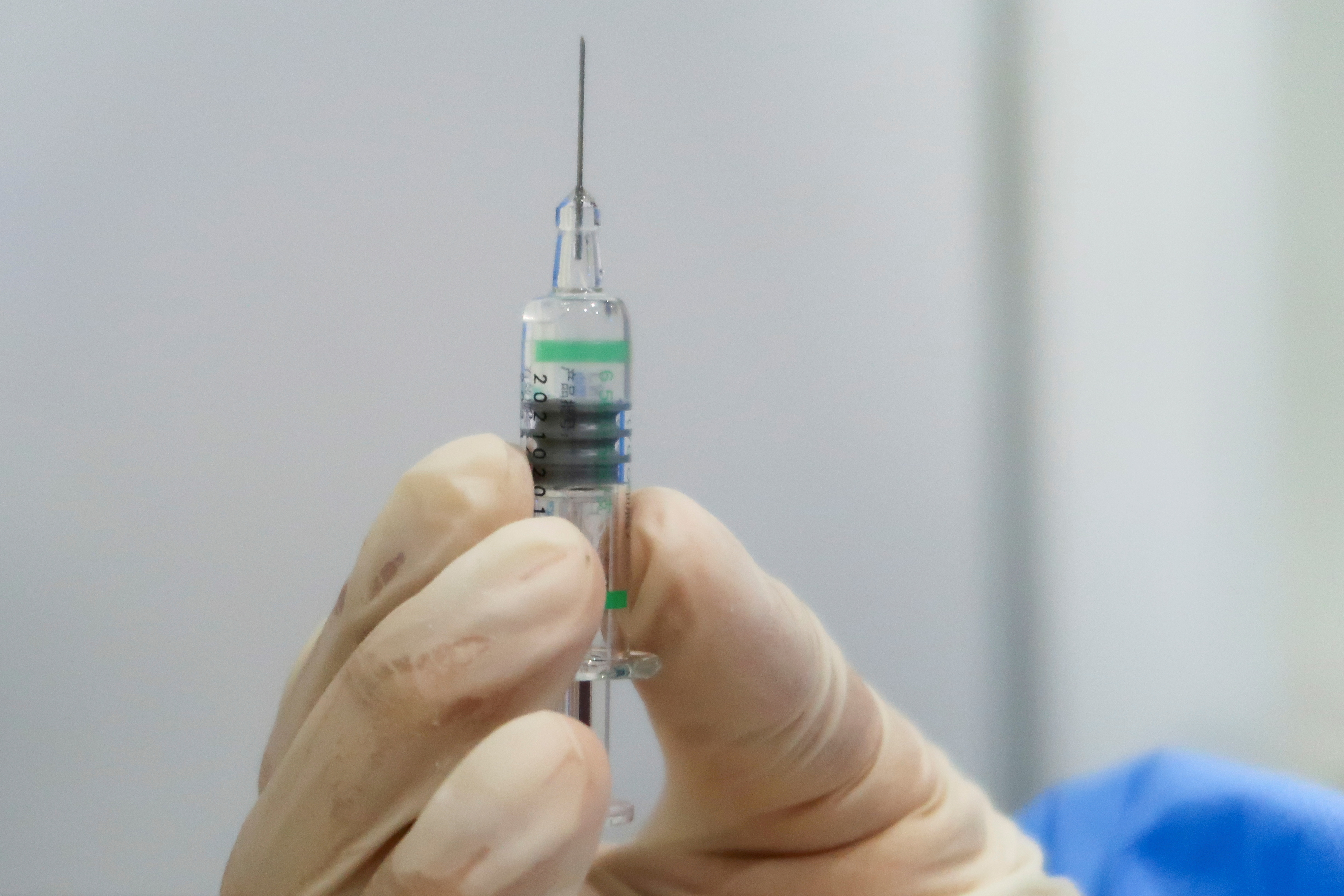
Uae Bahrain To Offer Sinopharm Covid 19 Booster Shots Reuters
Sinopharm Unit Inches Toward Vaccine Breakthrough Chinadaily Com Cn

Uae Launches Covid 19 Vaccine Production With China S Sinopharm Reuters
According to the WHO a large multi-country phase three. Those registered can already receive their first doses of the vaccine on Monday July 5.
Sinopharm Unit Inches Toward Vaccine Breakthrough Chinadaily Com Cn
What you need to know.

Sinopharm vaccine registration
. On Johnson Johnsons Janssen Covid-19 vaccine Dr Noor Hisham said the vaccine which is also known as Janssen Covid-19 Vaccine Suspension for Injection is manufactured by Belgium-based Janssen Pharmaceutica NV. Meanwhile Nithi Mahanonda CRA secretary-general posted on his Facebook page that another 1 million vaccine doses had been delivered to Thailand. Al Owais noted that the approved Sinopharm vaccine is the same vaccine that was used in the groundbreaking 4Humanity trials the first global clinical Phase III trial of an inactivated vaccine to combat COVID-19 which was launched in UAE in July this year and later was officially registered by the UAE Ministry of Health and Prevention MOHAP on 9th December. Of the 100 million Chinese vaccines half will be provided by Sinopharm and half by Sinovac with deliveries planned for July to September 2021 a WHO document dated July 29 says.Raffles Medical Group asked people to register their interest in the Sinopharm vaccine in a Facebook post on Monday. DUBAI Reuters - Bahrain said on Sunday it had approved a COVID-19 vaccine developed by China National Pharmaceutical Group Sinopharm and launched online registration for the vaccine for. Covid-19 vaccine is a mean to curtail. It is approved for use in those aged 18 and above.
WHO approves Sinopharm vaccine for emergency use in all countries. Sinopharm Vaccine Approved By HSA Under Special Access Route. The WHO issued its emergency use listing for the Sinopharm vaccine on May 7 2021 some 4 months after Chinas National Medical Products Administration authorized it on December 31 2020. The HSA has approved 11 applications for private hospitals and clinics to import Chinas Sinopharm vaccine under the special access route.
On 9 December 2020 Ministry of Health and Prevention MOHAP announced that it had officially registered the Beijing Institute of Biological Products inactivated vaccine known as Sinopharm vaccine which was developed jointly with Sinopharm -. Malaysias health ministry said on Friday Jul 16 it has granted conditional approval for emergency use to the COVID-19 vaccine manufactured by Chinas Sinopharm. Sinopharms COVID-19 vaccine. The registration period for the Sinopharm vaccine went from June 14 to June 18.
The Straits Times. He said Malaysias conditional registration approval for the use of the Sinopharm vaccine has been given to Duopharma M Sdn Bhd. The registration opened on Sunday at 8am through the website and CRA SINOP. CRAs Sinopharm jabs registration is aimed at 40000 citizens in 10 red zone provinces.
Here is what you need to know. The registration is available via bookingmohgovge as well as the health ministry hotline 1522. Sinopharm and Pfizer- BioNTech vaccines are available for citizens and residents for free Those who wish to get the should book prior appointment in one of the vaccination centers below. Over 14000 organisations register for Sinopharms vaccine The Chulabhorn Royal Academy CRA announced on Friday that 14634 organisations had applied to get Sinopharms Covilo vaccine to inoculate 4545272 staff as an alternative to the governments Sinovac and AstraZeneca vaccines.
COVID-19 Vaccination Updated Sinopharm Vaccine Guidelines Objective To provide guidelines for the Sinopharm vaccine storage handling administration and safe disposal along with recommendations for vaccine recipients. The Sinopharm COVID-19 vaccine. Under the special access route SAR Raffles Medical Group has received approval to import the Sinopharm vaccines. You may register your interest and you will be notified on next steps once the vaccine is available.
The CRA announced on Saturday June 19 that 17 070 organizations and companies nationwide have registered to obtain the Sinopharm vaccine to inoculate more than 487 million employees. The following groups can get the vaccine by visiting one of SEHAs vaccination centres without prior appointment. The Sinopharm vaccine is administered in two doses given three to four weeks apart. Download the COVID-19 Vaccine Pre-Registration Forms.
Even though the Pfizer-BioNTech and Moderna vaccines are available for free under our national vaccination programme some may look for alternative options. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group.

Uae Launches Covid 19 Vaccine Production With China S Sinopharm Reuters

Uae Bahrain To Offer Sinopharm Covid 19 Booster Shots Reuters

Vietnam Licenses Firm To Import 5 Mln Doses Of Sinopharm Vaccine Reuters

Covid 19 Taskforce Approves Vaccination For 3 11 Year Olds And Expands Vaccine Choice For Those Aged 12 17 The Official Website For The Latest Health Developments Kingdom Of Bahrain
/cloudfront-us-east-2.images.arcpublishing.com/reuters/K7IMX7V5NZOOVGSOPKVPNUA6MY.jpg)
Uae Bahrain To Offer Sinopharm Covid 19 Booster Shots Reuters

Uae Bahrain To Offer Sinopharm Covid 19 Booster Shots Reuters


Posting Komentar untuk "Sinopharm Vaccine Registration"